Artificial Light at Night: What You Need to Know
Today, I have a guest contribution from Examine.com researcher, Lucas Roldos. This is an adaptation of an Editor’s Pick from Examine (1), and it’s especially timely, as Examine is celebrating their 13th anniversary with some great sales HERE.
The study in question: Artificial light at night suppresses the day-night cardiovascular variability: evidence from humans and rats
The 24-hour light/dark cycle is the most important factor affecting our circadian rhythm. As our exposure to light changes throughout the day, a region of the brain called the “suprachiasmatic nucleus” responds by modifying the release of hormones (like cortisol and melatonin), body temperature, blood pressure, and mental alertness. This physiological responsiveness to light makes sure that we are alert and ready to take on the day when the sun is out, and that we are able to rest when the sun is down. Of course, the sun isn’t our only source of light anymore. Exposure to light sources like computer screens and light pollution can cause our bodies to “feel” like it’s daytime, even when it’s late at night.
This study (2) was a narrative review that investigated whether exposure to artificial light at night (ALAN) had any negative effects on cardiovascular health or cortisol levels in adults (some of whom were night shift workers).
The included studies differed slightly in their findings (and certain confounders were hard to control for), but generally speaking, the more ALAN exposure a person had, the stronger their risk for high blood pressure, heart rate, autonomic nervous system activity, and carotid artery intima-media thickness (which can indicate higher risk of subclinical atherosclerosis) (3), as well as lower day-night heart rate variability and disrupted cortisol levels.
We still need more research to confirm the details of this relationship, but it’s worth paying attention to these results. If ALAN is as problematic as it appears in this study, it would certainly make a good case for reducing exposure to things like smartphones, computers, and televisions at night. What’s more concerning, however, is the implications it has for areas with light pollution. The Earth’s artificial light area and brightness has been increasing by 2% per year in recent decades (4), and it’s estimated that 83% of the world’s population lives in areas that have light pollution. (5) It’s recommended that people avoid exposure to light intensities above 10 lux in the evening, and even basic light pollution from sources like streetlamps, cars, and building lights can easily exceed this amount. (6,7)
Data from Brown et al. (2022) (7), Gaston et al. (2013) (8), and Wood et al. (2013). (9)
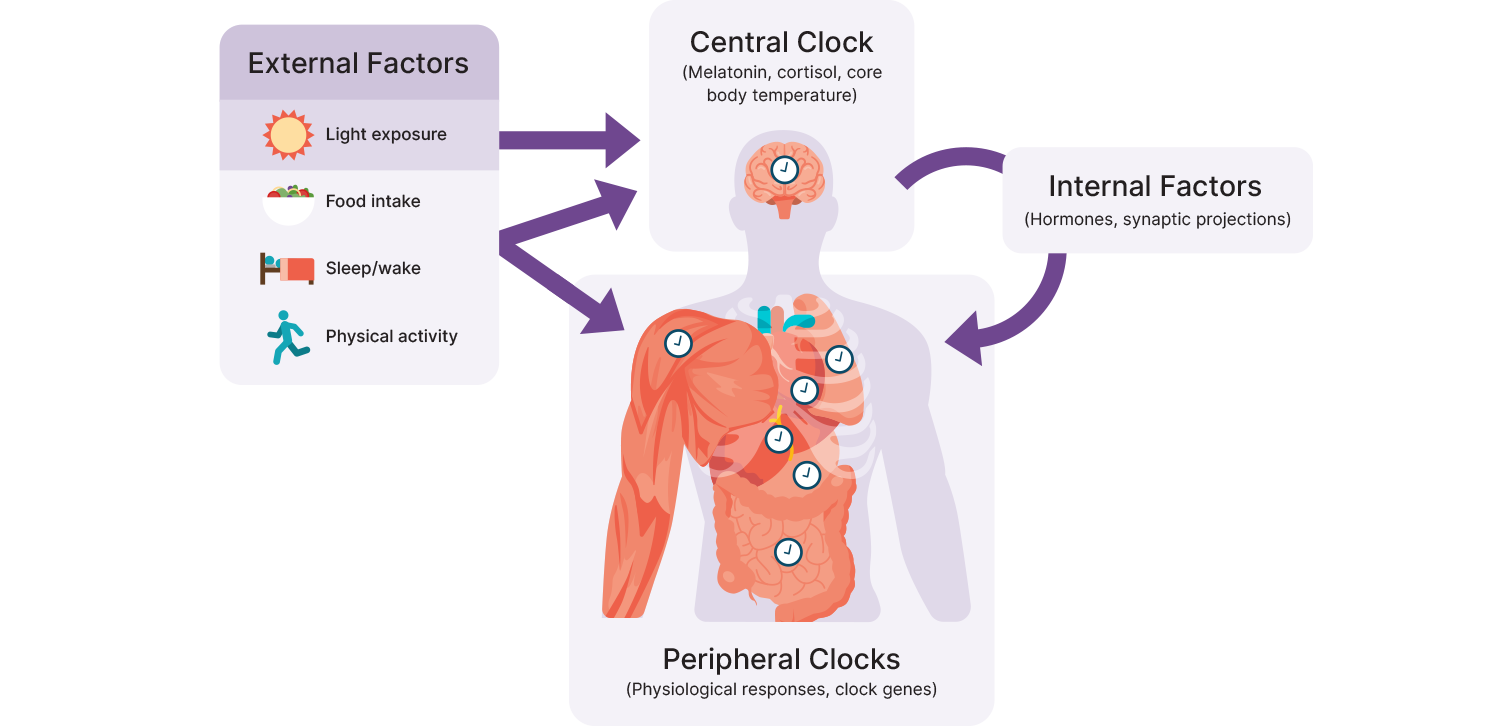
So how specifically does ALAN (and light generally) affect our circadian rhythms? As mentioned previously, the central pacemaker of the circadian system is the *suprachiasmatic nucleus* (SCN) found within the hypothalamus, which plays a role in the synchronization of almost all physiological activity (e.g., blood pressure, temperature, mental alertness).10 It is directly connected to the retina in the eye to ensure synchronization with the 24-hour light-dark cycle and projects to various internal “clocks”. Internal or local clocks are run by *transcriptional-translational feedback loop* (TTFL) mechanisms, which are essentially on/off switches that are triggered by certain cues, such as level of light exposure or quantity and timing of food intake, and generally oscillate between two states (e.g., wake/sleep, feed/fast).
The primary mechanism by which ALAN affects circadian rhythm is likely via the inhibition of [melatonin] secretion and downregulation of circadian-related genes involved in TTFLs that are both closely related to wake/sleep behavior. (11) In the specific case of cardiovascular disease, ALAN seems to inhibit melatonin secretion, which reduces mitochondrial recycling (mitophagy) and efficiency (e.g., fusion) (12), and circadian clock gene expression that disregulates the circadian rhythm and can lead to systemic inflammation and oxidative stress. Given that several cardiovascular parameters, like blood pressure and heart rate, are modulated by the circadian system and show clear 24-hour rhythms, it is not surprising that exposure to a cue that alters the rhythm will influence the cardiovascular parameter, especially when the external cue conflicts with internal clocks. (13) However, it should be noted that ALAN is not the only factor involved in circadian disruption, as exposure to cues like activity, temperature, or food intake outside of circadian rhythms could also contribute to circadian rhythm abnormalities and any associated health issues.
Adapted from Poggiogalle, Jamshed & Peterson, 2018. (14)
The general recommendation is to maintain consistent lifestyle habits and behavioral patterns that align with the circadian rhythm for better health, including timing of wake/sleep, feed/fast, and exertion/recovery, as well as light exposure, because life is full of events that will periodically disrupt the natural rhythm. (15) If consistency is the norm, a few acute stresses of ALAN or a late night snack should be straightforward for the body to recover from and shouldn’t lead to cardiovascular disease. For people with cardiovascular disease, ALAN management fits into stress reduction, along with sleeping well and avoidance of tobacco and alcohol within a bigger picture prevention/treatment plan that includes exercise, weight management, and dietary management. (16)
If you liked what you read here, then you’ll love the full offering at Examine.com. It’s my go-to resource for staying on top of the latest research relating to health and human performance. They’ve got a great 13th anniversary sale going on right now; you can check it out HERE.
Note: references for this article are posted as the first comment below.